
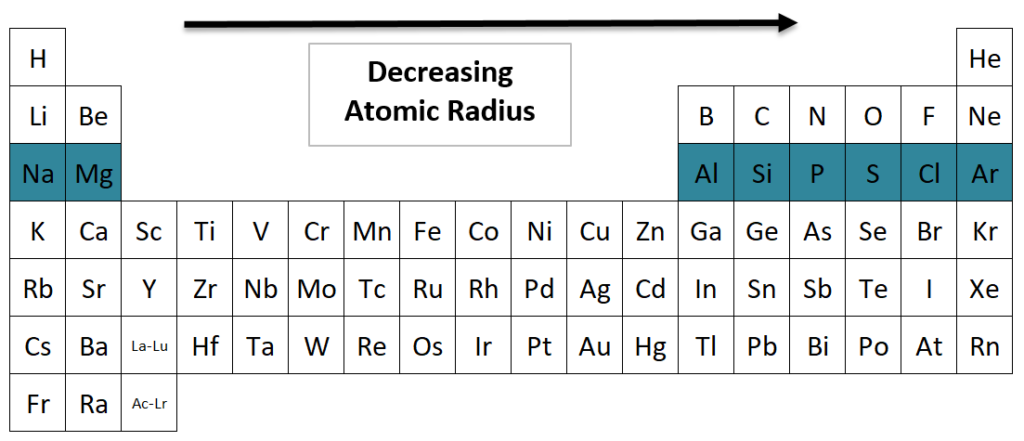
^ "Electron Affinity Trend | Science Trends"."Atomic shells according to ionization energies". ^ "Ionization Energy Trend | Science Trends".(ed.), "Chemistry at the Edge of the Periodic Table: The Importance of Periodic Trends on the Discovery of the Noble Gases and the Development of Noble-Gas Chemistry", The Periodic Table I: Historical Development and Essential Features, Cham: Springer International Publishing, pp. 157–196, doi: 10.1007/430_2019_49, ISBN 978-5-5, retrieved In contrast, the nonmetallic character decreases down the groups and increases across the periods. Across each period, from left to right, the increasing attraction between the nuclei and the outermost electrons causes the metallic character to decrease. Metallic properties generally increase down the groups, as decreasing attraction between the nuclei and outermost electrons causes these electrons to be more loosely bound and thus able to conduct heat and electricity.

The energies of these (n-1)d & ns orbitals are relatively close. These elements show variable valency as these elements have d-orbital as the penultimate orbital and s-orbital as the outermost orbital. However, this periodic trend is sparsely followed for heavier elements, especially for the F block and the transition metals. Hence, all the elements of a particular group have the same valency. However, as we move down in a group, the number of valence electrons does not change. But the valency of elements first increases from 1 to 4, and then it decreases to zero as we reach the noble gases. Trend-wise, while moving from left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. Electrons found in the outermost shell are generally known as valence electrons the number of valence electrons determines the valency of an atom. In simple terms, it is the measure of the combining capacity of an element to form chemical compounds. The valency of an element is the number of electrons that must be lost or gained by an atom to obtain a stable electron configuration. As a result, the force of attraction of the nucleus for the electrons increases and hence the electronegativity increases from aluminium to thalium. It is due to the fact that the atomic size increases as we move down the group, but at the same time the effective nuclear charge increases due to poor shielding of the inner d and f electrons. However, in group XIII ( Boron Family), the electronegativity first decreases from boron to aluminium and then increases down the group. However, if one moves down in a group, the electronegativity decreases as atomic size increases due to the addition of a valence shell, thereby decreasing the atom's attraction to electrons. Trend-wise, as one moves from left to right across a period in the modern periodic table, the electronegativity increases as the nuclear charge increases and the atomic size decreases. According to this scale, fluorine is the most electronegative element, while cesium is the least electronegative element. The scale has been named the Pauling scale in his honour. The most commonly used scale to measure electronegativity was designed by Linus Pauling. It is a dimensionless property because it is only a tendency. The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. In that case, the ionization energy decreases as atomic size increases due to adding a valence shell, thereby diminishing the nucleus's attraction to electrons. However, suppose one moves down in a group. The decrease in the atomic size results in a more potent force of attraction between the electrons and the nucleus. Trend-wise, as one moves from left to right across a period in the modern periodic table, the ionization energy increases as the nuclear charge increases and the atomic size decreases. The energy needed to remove the second electron from the neutral atom is called the second ionization energy and so on. The first ionization energy is the amount of energy that is required to remove the first electron from a neutral atom. It is also referred to as ionization potential. The ionization energy is the minimum amount of energy that an electron in a gaseous atom or ion has to absorb to come out of the influence of attracting force of the nucleus.


 0 kommentar(er)
0 kommentar(er)
